Molecular cooperativity is an overarching design concept that guides OncoNano’s efforts in oncology product development. The word “cooperativity” is used in scientific literature to describe inter-dependent multi-component biological processes where the whole is bigger than the sum of its parts. Inspired by this general phenomenon in nature, OncoNano aims to design therapeutic and imaging products that harness synergistic interactions among our polymers, payloads, and targeted biological systems to achieve enhanced efficacy and reduced toxicity.
Overview
OncoNano’s Technology Breakthrough: Polymeric Micelles that Target Tumor pH or STING Protein for Cancer Therapy
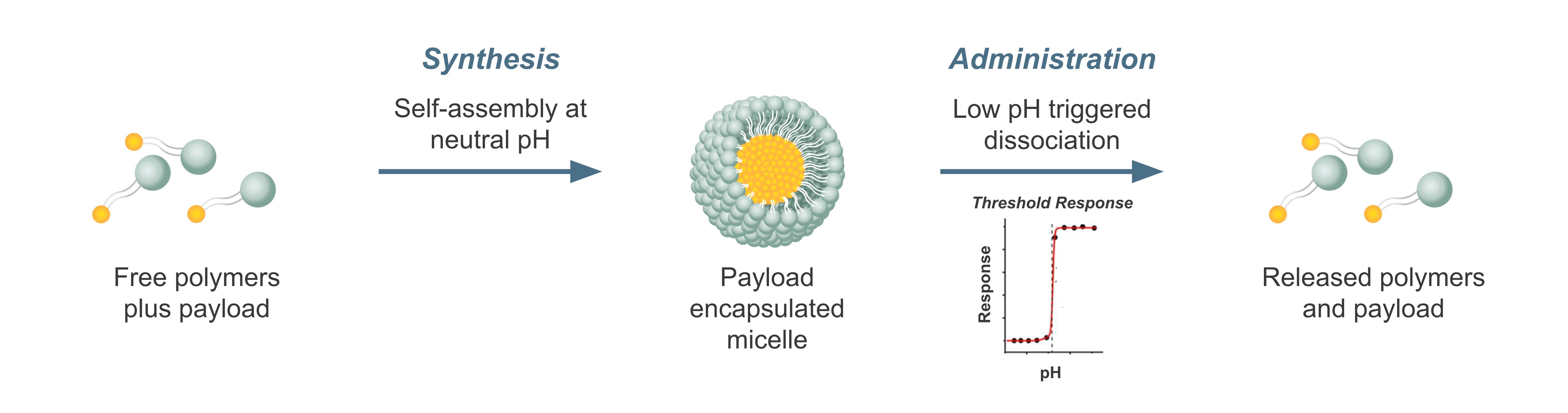
Tumor Activation. Molecular cooperativity governs micelle encapsulation by self-assembly and release of therapeutic payloads for targeted delivery to tumors, overcoming dose-limiting toxicities
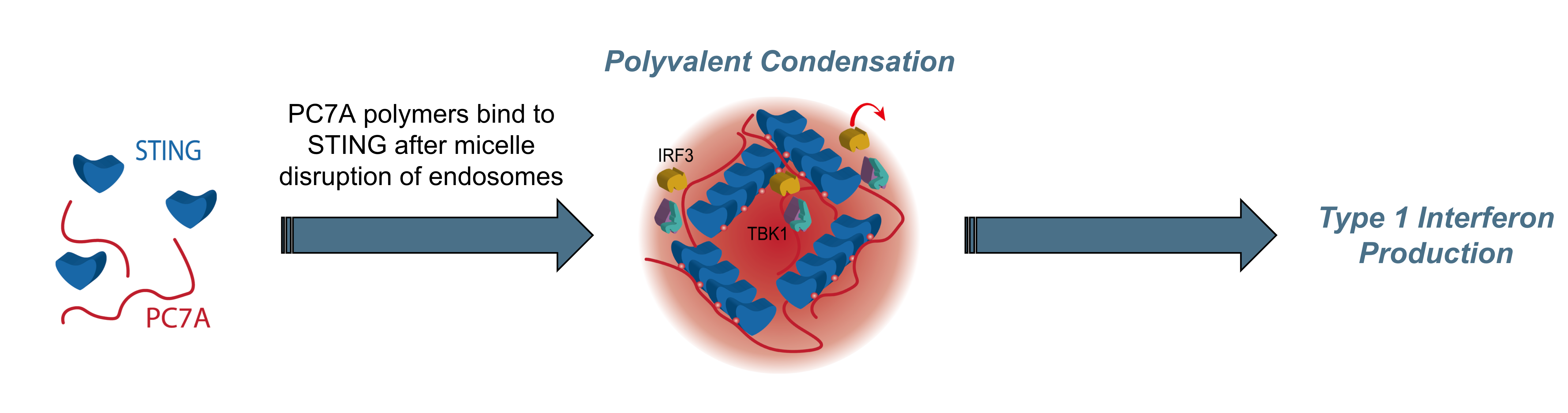
Immune Activation. Molecular cooperativity enables micelle disruption of endosomes to reach cytosolic STING target where released polymers and STING proteins form polyvalent condensates for robust immune activation against solid tumors
Powerful Technology Supports Two Platforms
Meaningful impacts across the continuum of patient care
ON-BOARD™
Delivery of Payloads to Target
Proprietary Product Candidate:
- Pegsitacianine intraoperative imaging agent (ONM-100) in Phase 2 clinical trials
- NIH/NCI SBIR and 2-time CPRIT grant awardees ($18MM total)
Key Strategic Partners for Therapeutic Delivery:
- Two research collaborations with big pharma companies
OMNI™
Polyvalent STING Activation
Proprietary Product Candidate:
- ONM-501: dual activation of STING by cGAMP and OMNI™ polymers yields robust T cell immunity against solid cancers
- CPRIT grant awardee ($15.4MM)
OncoNano intellectual property includes, among other rights and/or assets, patents
exclusively licensed from The University of Texas Southwestern Medical Center.
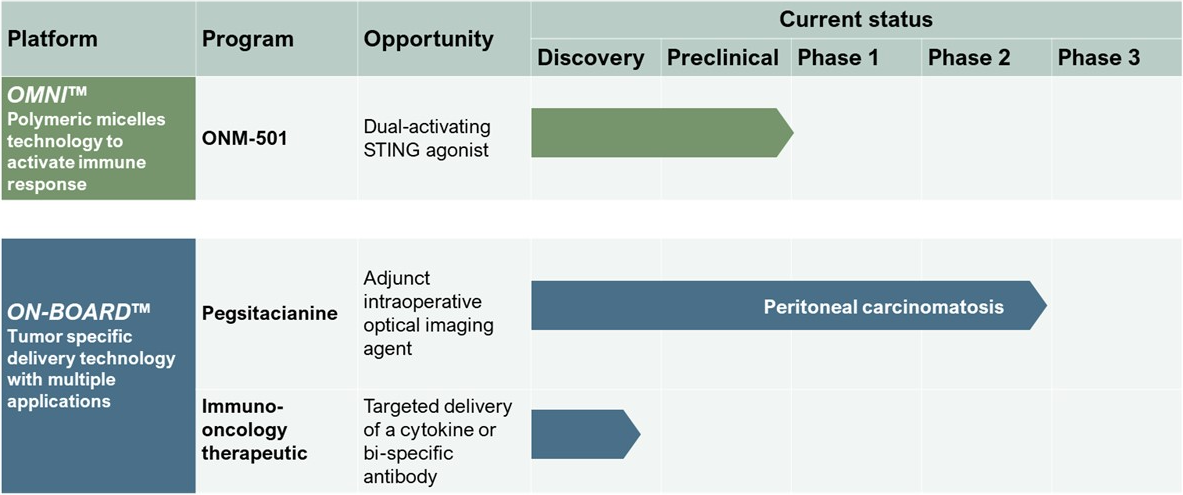
Pipeline
Differentiated Pipeline to Benefit Cancer Patients
ON-BOARD™
The ON-BOARD™ micelle platform is designed to carry a wide variety of payloads for systemic administration to the tumor microenvironment. Micelles can be tuned to accommodate small molecules and biologics such as antibodies and cytokines with a design that enables improved tumor specific accumulation compared to other polymeric drug delivery approaches. Payloads can be physically encapsulated or chemically bound to the micelle’s polymeric constituents, providing protection of the payload during transit to its target and minimization of systemic exposure and toxicity. The simplicity of the micelle approach eliminates the need for more complex and active targeting strategies such as mAbs that limit therapeutic utility. OncoNano is currently engaged in two research collaborations with large pharma companies to develop ON-BOARD therapeutic products and is actively pursuing additional ones.
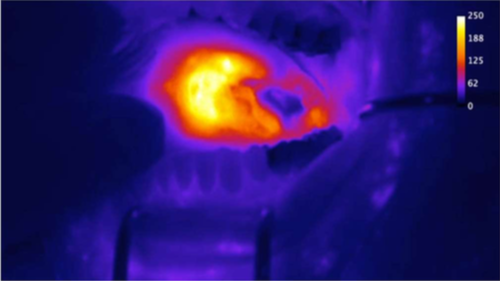
Our lead imaging agent, Pegsitacianine (previously ONM-100), is a fluorescence-based IV injectable for use in image-guided surgery. Phase 1 and on-going Phase 2 trials have shown Pegsitacianine detection of breast, head & neck, colorectal, esophageal, ovarian, prostate, and peritoneal metastatic cancers, continued favorable safety profile, and evidence of tumor-positive margins or occult disease detection that was missed by standard of care. Pegsitacianine is expected to function in all solid cancers, use commercial surgical cameras, and fit into existing surgical workflows – features that greatly facilitate adoption. The development of Pegsitacianine has been supported by two grants from the Cancer Prevention and Research Institute of Texas (CPRIT) and an SBIR grant from NIH/NCI.
OMNI™
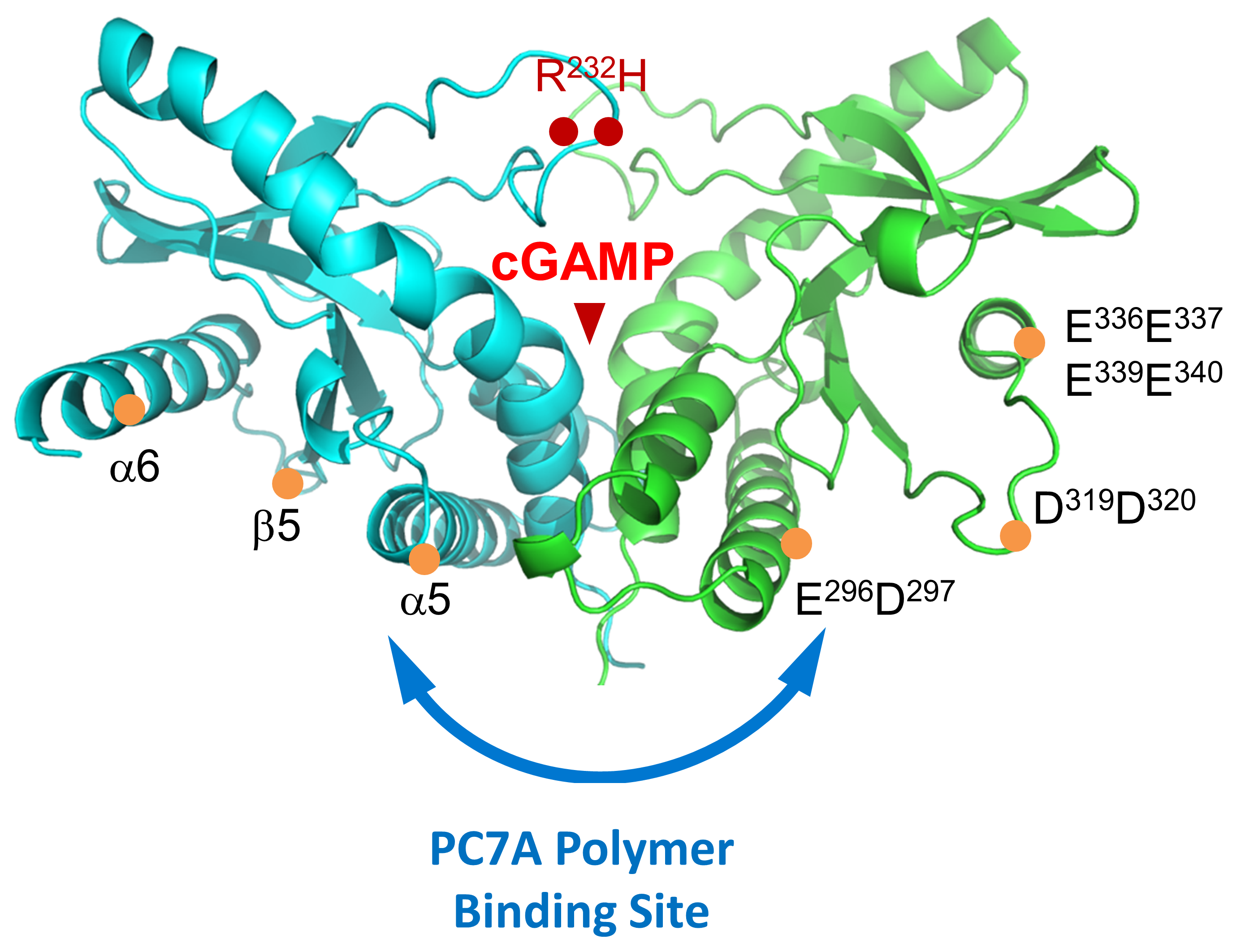
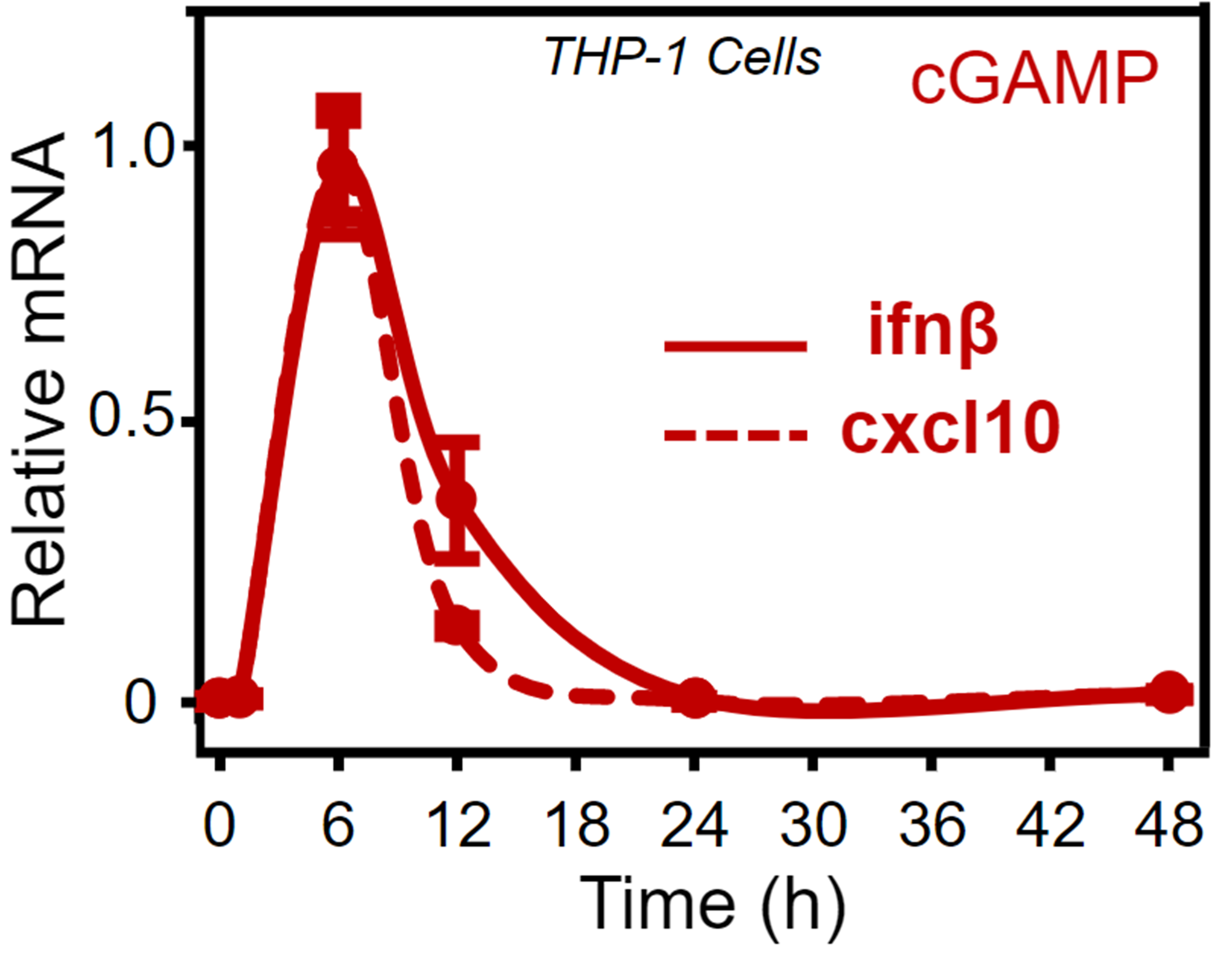
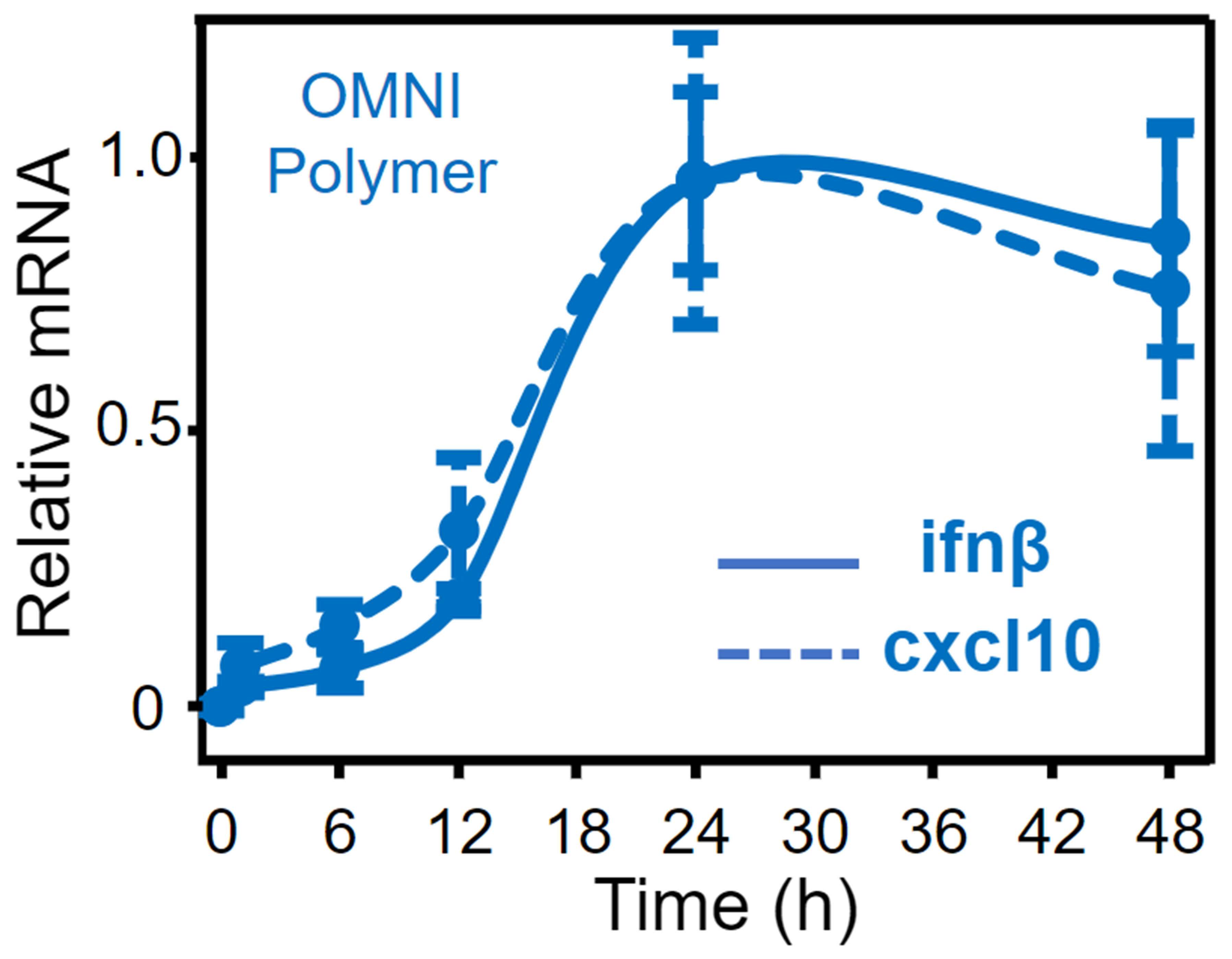
OncoNano’s OMNI™ platform further highlights versatility of the core micelle technology to not only deliver therapeutic payloads but also directly activate a ubiquitous immune signaling molecule for cancer immunotherapy. OMNI is composed of proprietary polymers tailored to activate Stimulator of Interferon Genes (STING) which plays a crucial role in the generation of T cell immunity against solid tumors. OMNI polymers interact in a new and differentiating manner compared to small molecule STING agonists by not only binding to a complementary site on the STING protein but via polyvalent interactions: binding of multiple proteins to each polymer chain. Compared to small molecules that activate STING for 6-8 hours, OMNI’s polyvalent interactions result in condensation of OMNI-STING aggregates that stabilize STING activation for up to 48 hours which is important to induce infiltration of immune cells to turn the cold tumors into hot ones by immunotherapy.
OMNI polymers can encapsulate payloads such as STING agonists and tumor-specific antigens to produce drugs that are designed to be endocytosed where the low endosomal pH environment triggers micelle dissociation and release of both the payload and OMNI unimers within the cytosolic compartments of dendritic cells to illicit a T cell immune response. Our lead product candidate, ONM-501, is a preclinical phase novel immunotherapeutic for intratumoral administration comprised of OMNI encapsulated cGAMP, an endogenous small molecule STING agonist. The complementary combination of an initial burst STING activation by cGAMP and sustained STING activation by OMNI results in a robust STING activation profile that is not possible with either component alone. ONM-501 shows antitumor efficacy and when combined with checkpoint inhibitors such as anti-PD-1 produces a synergistic tumor killing effect in multiple cancer types. Preclinical data also shows evidence that ONM-501 activates CDN-resistant STING variants in 20% of patients and a low probability for off-target toxicities including cytokine storms.
The development of ONM-501 is supported by a grant from the Cancer Prevention and Research Institute of Texas (CPRIT).
Expanded Access Policy
At OncoNano Medicine, we are committed to developing products that bring new, innovative therapies, like pegsitacianine (ONM-100), to patients with serious or life-threatening illnesses or conditions. OncoNano is developing pegsitacianine to improve the ability of surgeons to identify cancerous tissue in the peritoneum during cytoreductive surgery.
At this time, OncoNano does not offer an expanded access program and is not accepting expanded access requests for investigational products, such as pegsitacianine.
OncoNano’s current focus and priority is to complete the product development program for its investigational product(s), such as pegsitacianine, in order to obtain the required safety and efficacy data needed for regulatory approval. We believe that focusing our resources on our clinical trial program is the best path forward to bring our investigational product(s) to patients as quickly and safely as possible.
As we continue to develop investigational product(s), we will review our expanded access policy for investigational product(s) and may make updates to this policy.
Patients can gain access to our investigational product(s), such as pegsitacianine, by participating in a clinical trial. If you or someone you know would like to learn more about OncoNano Medicine’s clinical trials, we encourage you to view our trials at www.clinicaltrials.gov.
If you have additional questions about OncoNano’s expanded access policy, please email us at info@onconanomed.com.